Muzi Yan
CHE 131-b
Professor Young
16/5/1
The Chemistry of Fireworks, Colored glass, Color and Atom
Summary of Articles
The first article is about the chemistry of firework.

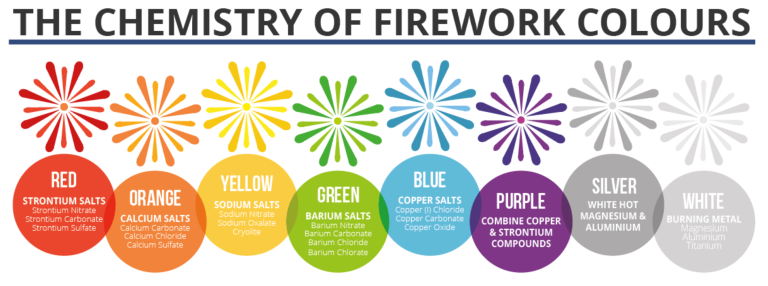
The article mainly talks about five basic components of firework – gunpowder, binder, oxidizer, chlorine donors and color producers.
First, the history and combination of gunpowder are introduced. Gunpowder contains sulfur(S), potassium nitrate (KNO3), and charcoal(C). Serving as a fuel, gunpowder allows fireworks to burn, providing energy to this beautiful visual enjoyment.
Second, the author talks about the binder, which is used to hold the mixture of the components together. The most commonly used binder is dextrin, one of the organic compound.
Then the oxidizer, which provides oxygen for the combustion of gunpowder, is introduced. The oxidizers are usually nitrates, chlorates or perchlorates.
The fourth part of firework is chlorine donors, which are chlorate and perchlorate. They can help to strengthen the colors of firework.
The fifth component of firework is color producers – salts, which are the reason why we get to enjoy all the different and amazing colors of firework. Different types of salts can produce different intense color when burned.
The second article is about the chemistry of colored glass.

nerdreactor.com
First, it talks about the chemical composition of two types of glass. Soda-lime glass is the type of glass which we use in daily life. It contains a mixture of silicon dioxide, calcium oxide (lime) and sodium oxide (soda). The second type is borosilicate glass. This type of glass contains boron trioxide as well as silicon dioxide. The greater durability, greater chemical and heat resistance of borosilicate glass leads to its use in laboratories.
The article also talks about three main methods to produced colored glass. By the way, I think this technique should be counted as one of the greatest inventions in art field. After browsing several pictures of some gorgeous stained glass art piece, I believe one of the best way to describe glass art is that – “imagine a beautiful painting that you like. Now imagine the painting with sunlight streaming through. That’s essentially what glass arts look like.”

nerdreactor.com
The first method to produce colored glass is to introduce transition metals or rare earth metal oxides to the glass.
The second method is the formation of colloidal particles – particles of a substance that are suspended throughout the glass.
The third method is to add the already colored particles to glass.
Connections of Articles
The biggest connection of these two articles is that they both explain how we get to see the different colors of objects. For firework, the reason is the metal ions contained in salts. As we learned in the chemistry class, the heat released by the combustion causes electrons in the metal atoms to be excited and go to higher energy levels. Because the excited states are unstable, the electrons will finally fall back to its ground state, emitting the previous absorbed energy as light. Each metal atom has a different energy gap between the ground state and excited state, causing the difference of color emitted. This explanation can also be applied to the flame tests which professor Fieberg did on the chemistry lab.

cshakira1.blogspot.com
For glass, the reason why the introduce of transition metals and rare earth metal oxides can give color to glass is that different metal ions can absorb certain wavelengths of light, causing the glass has different colors. The second method, the formation of colloidal particles, can produce colored glass is because the colloidal particles scatter light of particular frequencies as it passes through the glass, leading to the different-colored glass.
Both articles relate the different color of objects with the absorption and release of different-frequency light of different atoms. For example, in both cases of firework and colored glass, copper ion can lead to the color blue because it will only absorb certain amount of energy and release it at the wavelength of blue light.
Relationship with the Third Topic
The third topic I come up with is the chemistry of color and atom. After atoms’ electrons absorbing energy (by heat, light, etc), they are excited and rise to higher energy levels.
However, because nature tends to favor the more stable, low-energy states, the electrons will finally fall back to their ground states, emitting the previous absorbed energy. In the firework and colored glasses cases we previously discussed, the energy is released as light. In addition, because different atoms have different energy gaps between their ground states and excited states, different wavelengths of light will be emitted, causing different colors to appear.
martinkeogh.com
Cited
http://www.compoundchem.com/2015/03/03/coloured-glass/
http://www.compoundchem.com/2013/12/30/the-chemistry-of-fireworks/